Johnson & Johnson Vaccine Distribution Pauses Over Blood Clot Concerns
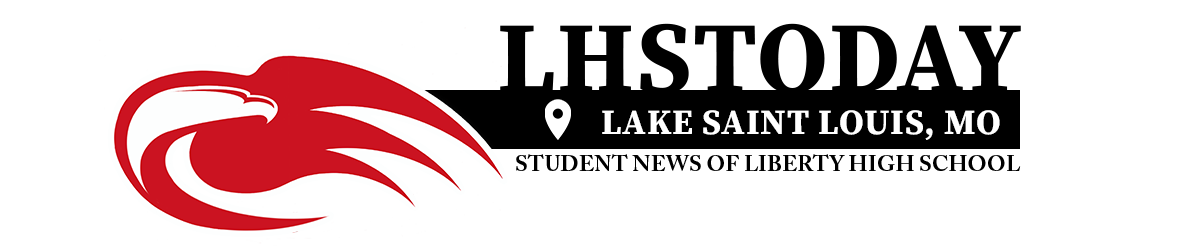
Phil Long/Associated Press via The New York Times
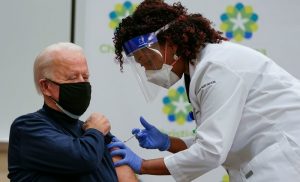
A Kent State University student getting his Johnson & Johnson vaccination in Kent, Ohio, last week.
April 13, 2021
Use of the Johnson & Johnson COVID-19 vaccine has been halted after six blood clot cases that were tied to the vaccine have arisen. The Food and Drug Administration and the Centers for Disease Control have recommended that this brand of vaccine stop being used at federal and state sites.
There have been six reported cases of this “rare and severe” type of blood clot, all six cases occurring in women between the ages 18-48. The women reported that the blood clot developed 1-3 weeks after getting the shot. One of the women has died, while another woman has been hospitalized in critical condition.
So far, there have been around 6.8 million administered doses of the Johnson & Johnson vaccine in the country. According to the CDC, around nine million more doses have been shipped to the United States; however Johnson & Johnson released a statement Tuesday announcing that they are going to delay the rollout of its vaccine in Europe.
The statement also relays the companies attitude towards the situation, “We have been working closely with medical experts and health authorities, and we strongly support the open communication of this information to healthcare professionals and the public.”
Dr. Anne Schuchat, principal deputy director of the CDC, and Dr. Peter Marks, director of the FDA’s Center for Biologics Evaluation and Research released a joint statement on Tuesday saying, “CDC will convene a meeting of the Advisory Committee on Immunization Practices (ACIP) on Wednesday to further review these cases and assess their potential significance… the FDA will review that analysis as it also investigates these cases. Until that process is complete, we are recommending a pause in the use of this vaccine out of an abundance of caution. This is important, in part, to ensure that the health care provider community is aware of the potential for these adverse events and can plan for proper recognition and management due to the unique treatment required with this type of blood clot.”
During the Tuesday news conference, the acting commissioner of the FDA noted that the pause on administering the Johnson & Johnson vaccine should only last a few days., and that the time frame depends on “what we learn in the next few days.”
In the meantime it is recommended that if you are eligible, to get the Pfizer and Moderna vaccines.